| Method no.: | 90 |
| Matrix: | Air |
| Target concentration: | 1 ppm (6 mg/m3) |
| Procedure: | Samples are collected by drawing air through an open-face sampling device containing two glass fiber filters, each coated with 10 mg of 3,4-dimethoxybenzylamine (veratrylamine). Samples are extracted with 90:10 (v/v) acetonitrile/dimethyl sulfoxide and analyzed by HPLC using a UV detector. |
| Recommended air volume and sampling rate: |
75 L at 1.0 L/min |
| Reliable quantitation limit: | 0.008 ppm (0.048 mg/m3) |
| Standard error of estimate at the target concentration: (Section 4.7.) |
5.5% |
| Special requirement: | Store samples in a refrigerator upon receipt at the laboratory. |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: October 1991 | Chemist: Yihlin Chan |
OSHA Salt Lake Technical Center
Salt Lake City, UT 84165-0200
1. General Discussion
- 1.1. Background
- 1.1.1. History
For determining the workplace exposure to phthalic anhydride in air, NIOSH recommends collection with cellulose ester membrane filters, extraction with dilute aqueous ammonia, and analysis by HPLC (Ref. 5.1.). Others have collected phthalic anhydride on glass fiber filters and extracted with dilute aqueous sodium hydroxide (Ref. 5.2.). Because both these methods convert the collected phthalic anhydride to, and analyze as, phthalic acid, they are susceptible to positive interference from any phthalic acid originally present in the air. Moreover, filters are not effective in trapping vapor and fume. Vapor as well as aerosol is generated in industrial phthalic anhydride processes which typically take place at elevated temperatures. Pfaeffli used a Tenax tube connected downstream in series with a membrane filter for collecting any vapor that had penetrated the filter (Ref. 5.3.). He desorbed the filter and the adsorbent with methyl t-butyl ether and analyzed directly for the anhydride with GC/ECD to eliminate the interference from phthalic acid present during sampling. But the possibility of the collected anhydride being partially hydrolyzed to phthalic acid before analysis still remains.
OSHA has been collecting phthalic anhydride in isopropanol impingers and analyzing the resulting half ester by HPLC. This not only eliminates the interference from the phthalic acid originally present in the air, but also prevents the loss of anhydride through hydrolysis after it has been collected. However, the sampling technique is cumbersome and potentially inefficient.
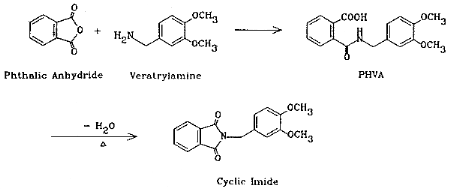
The method presented here overcomes the above difficulties by the use of coated filters for collection. Phthalic anhydride is collected with veratrylamine-coated glass fiber filters where the anhydride is derivatized in situ to form an acid-amide (phthalamic acid). The reaction is fast and quantitative. Although cyclic anhydrides are easily converted to cyclic imides with amines via intermediate halfamides (Ref. 5.4.), the product in this case is the half amide (PHVA), not the cyclic imide. This was confirmed by the independent synthesis of the cyclic imide and the comparison of the two infrared spectra (Section 4.13.). Phthalic acid does not form this derivative so it does not interfere. The derivative is stable and has a high UV absorptivity, the latter contributing to the high sensitivity of the analytical procedure.
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Phthalic anhydride in the form of vapor, fume, or dust irritates the eyes. It is both an irritant and sensitizer of the skin and respiratory tract, and may produce asthma on repeated exposure. Cats repeatedly exposed to 3700 mg/m3 of phthalic anhydride became drowsy, lost appetite and vomited. Liver and kidney injury occurred. Air concentrations of 30 mg/m3 caused conjuctivitis in workers. At 25 mg/m3 there were signs of mucous membrane irritation. Workers exposed to undetermined concentrations of mixed vapors of phthalic acid and phthalic anhydride developed conjuctivitis, bloody nasal discharge, atrophy of the nasal mucosa, hoarseness, cough, occasional bloody sputum, and bronchitis. Several cases of bronchial asthma resulted. There was skin sensitization with occasional urticaria and eczematous response. Phthalic anhydride is a direct but delayed irritant of the skin. It is more severely irritating in the presence of water, due to the pronounced effects of the phthalic acid which is formed. Prolonged or repeated exposure also may cause dermatitis. (Ref. 5.5.)
The ACGIH assigned a TLV-TWA of 1 ppm for phthalic anhydride (Ref. 5.6.). There is no STEL. The OSHA PEL-TWA is also set at 1 ppm (Ref. 5.7.).
1.1.3. Workplace exposure
The following list includes some common operations where exposure to phthalic anhydride may occur (Ref. 5.5.):
- (1) use in manufacture of plasticizers for use in polyvinyl
chloride, polyvinyl acetate, copolymer resins, cellulosic
plastics, alkyd resins, and non-drying oils or natural resins to
enhance properties; manufacture of unsaturated polyester resins
for use in structural building parts, swimming pools, automotive
parts, and luggage
(2) use in synthesis of dyes; use in manufacture of chemicals and chemical intermediates for production of insecticides, insect repellents, chemical reagents, urethane polymers, perfumes, and weed killers
(3) use in manufacture of pharmaceuticals and pharmaceutical intermediates; manufacture of metallic and acid salts; manufacture of epoxy resins as curing and hardening agents
(4) use in manufacture of fire retardants for use as components of polyester resins
1.1.4. Physical properties and other descriptive information (Ref. 5.8. unless noted otherwise)
| chemical name: | phthalic anhydride |
| CAS no.: | 85-44-9 |
| synonyms: | |
| structure: | 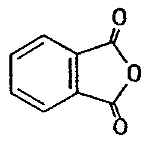 |
| molecular wt: | 148.12 |
| boiling point: | 284.5°C sublimes (Ref. 5.9.) 295°C sublimes (Ref. 5.10.) |
| melting point: | 131°C |
| vapor pressure: | 6.7 Pa (0.05 mmHg) at 20°C |
| flash point: | 152°C (closed cup) |
| appearance: | white crystalline solid |
| solubility: | soluble in acetone, MEK; slightly soluble in ether |
| Derivative (Ref. 5.11.) | |
| chemical name: | N-(3,4-dimethoxybenzyl)phthalamic acid |
| synonyms: | |
| structure: | 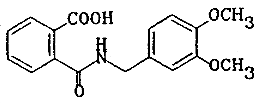 |
| molecular wt: | 315.33 |
| appearance: | white crystalline solid |
| melting point: | 120.0-121.5°C |
| solubility: | soluble in chloroform, methanol, acetonitrile, dimethyl sulfoxide (DMSO) |
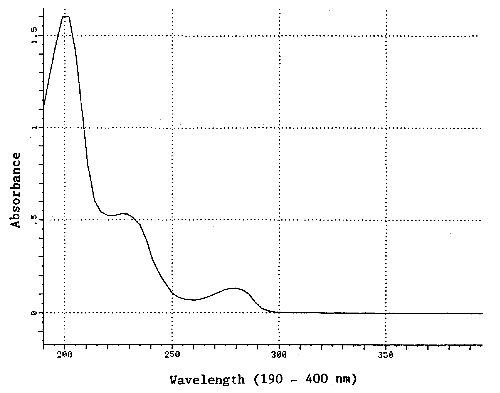
| UV spectrum: | Figure 1.1.4. |
The analyte air concentrations throughout this method are based on the recommended sampling and analytical parameters. Air concentrations listed in ppm are referenced to 25°C and 101.3 kPa (760 mmHg). The analyte concentrations are listed as those of phthalic anhydride even though the derivative is the actual species analyzed.
- 1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 2.5 ng per injection (5-µL injection of a 0.5 µg/mL solution). This is the amount of analyte which gave a peak whose height is approximately 5 times the height of baseline noise. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 2.34 µg per sample (0.005 ppm, 0.031 mg/m3). This is the amount of analyte spiked on the sampling device that, upon analysis, produces a peak similar in size to that of the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit (RQL) is 3.59 µg per sample (0.008 ppm, 0.048 mg/m3). This is the smallest amount of analyte spiked on the sampling device which can be quantitated within the requirements of a recovery of at least 75% and a precision (±1.96 SD) of ±25% or better. (Section 4.3.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of the analyte. When the target concentration of the analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Instrument response to the analyte
The instrument response is linear over the concentration range of 0.5 to 2 times the target concentration. (Section 4.4.)
1.2.5. Recovery
The recovery of the phthalic anhydride-veratrylamine derivative (PHVA) from the samples used in a 15-day storage test remained above 80% when the samples were stored at ambient temperature. The recovery remained quantitative after 15 days when the samples were stored at 0°C. (Section 4.5.)
1.2.6. Precision (analytical procedure)
The pooled coefficient of variation obtained from replicate injections of analytical standards at 0.5, 1, and 2 times the target concentration is 0.015. (Section 4.6.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the ambient 15-day storage test is ±10.8%. (Section 4.7.) This includes ±5% for sampling error.
1.2.8. Reproducibility
Six samples prepared by liquid spike and a draft copy of this procedure were given to a chemist unassociated with this evaluation. The samples were analyzed after 11 days of storage at 0°C. No individual sample result deviated from its theoretical value by more than the precision reported in Section 1.2.7. (Section 4.8.)
1.3. Advantages
Phthalic anhydride is derivatized in situ, eliminating the hydrolysis problem during storage. There is no interference from phthalic acid. The sampling procedure is simple and convenient.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. A personal sampling pump that can be calibrated to within
±5% of the recommended flow rate with the sampling device in line.
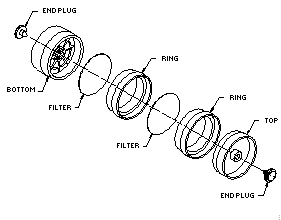
2.1.2. A three-piece polystyrene cassette containing two glass fiber filters, each coated with 10 mg of veratrylamine, and assembled as shown in Figure 2.1.2.
The coated filters are prepared by applying 0.4 mL of a freshly prepared solution of 25 mg/mL veratrylamine in methanol to each glass fiber filter and allowing them to dry in a hood or under vacuum. The coated filters should be stored in a closed container in a refrigerator and be used within a month after preparation.
2.2. Reagents
No reagents are required for sampling.
2.3. Sampling technique
- 2.3.1. Prepare the sampler for open-face sampling by removing
the top piece and the end plug from the bottom piece. Attach the
sampler to the sampling pump with a piece of flexible tubing and
place it in the worker's breathing zone with the open face of the
cassette facing down.
2.3.2. Replace the top piece and the two end plugs after sampling. Seal the sample end-to-end with an official OSHA seal (Form 21).
2.3.3. Submit at least one blank with each set of samples. Handle the blank the same as the other samples except draw no air through it.
2.3.4. List any potential interferences on the sample data sheet.
2.3.5. Submit the samples to the laboratory for analysis as soon as possible after sampling. If samples can not be shipped the same day, store at reduced temperature.
2.4. Sampler capacity
Sampler capacity was found to increase with increasing humidity. Sampler capacity measured at 10% RH, 25°C, and 2 times the target concentration exceeds 100 L. (Section 4.9.)
2.5. Extraction efficiency and stability of extracted samples (Section 4.10.)
- 2.5.1. The average extraction efficiency for PHVA from six
veratrylamine-treated glass fiber filters at the target
concentration was 99.1%.
2.5.2. Extracted samples remain stable for at least 1 day, with an average recovery of 98.2%.
2.6. Recommended air volume and sampling rate
- 2.6.1. Collect 75 L at 1.0 L/min.
2.6.2. For short-term sampling, collect 15 L at 1.0 L/min.
2.7. Interferences (sampling)
Excessive amounts of compounds that can react with veratrylamine (such as isocyanates, acid chlorides, and anhydrides other than phthalic) may reduce the sampler capacity by consuming part of the derivatizing agent.
2.8. Safety precautions (sampling)
Attach the sampling equipment to the worker in such a manner that it will not interfere with work performance or safety. Follow all safety practices applicable to the work area.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. An HPLC equipped with a UV detector. A Waters 600E pump,
900 photodiode array detector, and WISP autosampler were used in
this evaluation.
3.1.2. An HPLC column capable of separating veratrylamine, PHVA, and any interferences. A Waters Radial-Pak 100-mm × 8-mm i.d. cartridge containing Nova-Pak C18 (end-capped 5-m spherical particles) was used in conjunction with a Waters RCM-100 Radial Compression module.
3.1.3. An electronic integrator or other suitable means of measuring detector response. A Waters 860 Networking Computer System was used in this evaluation.
3.1.4. Sample vials, 4-mL glass, with Teflon-lined septum caps. WISP vials were used in this evaluation.
3.1.5. Volumetric flasks and pipets.
3.1.6. A mechanical shaker. A Fisher Roto-Rack was used in this evaluation.
3.2. Reagents
- 3.2.1. Phthalic anhydride-veratrylamine derivative (PHVA).
Synthesized and purified as in Section 4.12.
3.2.2. Extraction solvent: acetonitrile/dimethyl sulfoxide 90:10 (v/v). Both solvents were b&j Brand High Purity Solvent grade and were obtained from Baxter.
3.2.3. Phosphoric acid. Phosphoric acid, Baker Analyzed Reagent grade, was obtained from J T Baker.
3.2.4. Water, HPLC grade. The water was obtained from a Millipore Milli-Q water purification system.
3.3. Standard preparation
- 3.3.1. Prepare stock standards by weighing 10-20 mg of PHVA in
10-mL volumetric flasks and diluting to volume with the extraction
solvent. Apply a factor of 0.4697 to the weight of PHVA to convert
it to that of phthalic anhydride. For example, 10 mg of PHVA
dissolved in 10 mL will give a standard stock solution representing
0.4697 mg/mL or 469.7 µg/mL of phthalic anhydride.
(MW phthalic anhydride)/(MW PHVA) = 148.12/315.33 = 0.4697
3.3.2. Prepare analytical standards by diluting the stock standards with the extraction solvent. An analytical standard of 112.5 µg/mL (450 µg/sample) represents a target concentration sample.
3.3.3. Prepare a sufficient number of standards to generate a calibration curve. Analytical standard concentrations must bracket sample concentrations.
3.4. Sample preparation
- 3.4.1. Transfer the two filters to separate WISP vials. This is
best accomplished by double-folding the filter with the folds
parallel.
3.4.2. Add 4.0 mL of the extraction solvent to each vial.
3.4.3. Cap the vials and shake them on a mechanical shaker for 1 h.
3.5. Analysis
- 3.5.1. HPLC conditions
| column: | Nova-pak C18 (8 mm × 100 mm, 5-µ particle size) |
| eluent: | water/acetonitrile/phosphoric acid 73:27:0.1 (v/v/v) |
| flow rate: | 2.0 mL/min |
| injection volume: | 5 µL |
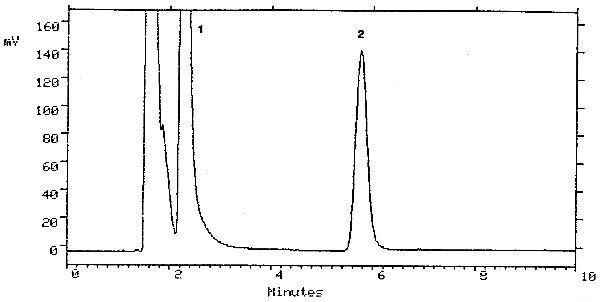
| retention time: | 5.8 min |
| chromatogram: | Figure 3.5.1. |
| UV detector: | 200 nm |
3.5.2. Construct a calibration curve using an external standard method by plotting micrograms per milliliter versus detector response of standards.
3.6. Interferences (analytical)
- 3.6.1. Any compound that absorbs at 200 nm and has a similar
retention time as PHVA is a potential interference. Generally,
chromatographic conditions can be altered to separate an
interference.
3.6.2. Retention time on a single column is not considered proof of chemical identity. Additional means of identification include: GC/MS, analysis using an alternate HPLC column, detection at another wavelength, and comparison of absorbance response ratios.
3.7. Calculations
The analyte amount for samples is obtained from the calibration curve in terms of micrograms per milliliter uncorrected for extraction efficiency. The back filter is analyzed primarily to determine if there was any breakthrough from the front filter during sampling. If a significant amount of analyte is found on the back filter (e.g., greater than 25% of the amount found on the front filter), this fact should be reported with sample results. If any analyte is found on the back filter, it is added to the amount found on the front filter. The analyte amount is then corrected by subtracting the amount found in the blank. The air concentration is obtained by using the following equations.
| mg/m3 = | (µg/mL) (4 mL)
(liters of air sampled) (extraction efficiency) |
| ppm = | (mg/m3) (24.46)
(148.12) |
| where | 24.46 | = | molar volume (liters) at 101.3 kPa (760 mmHg) and 25°C |
| 148.12 | = | molecular weight of phthalic anhydride |
3.8. Safety precautions (analytical)
Avoid skin contact and inhalation of all chemicals. Restrict the use of all chemicals to fume hood when possible. Wear gloves, safety glasses and a lab coat when working with chemicals.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 2.5 ng (5-µL injection of a 0.5 µg/mL solution). This is the amount of analyte that will give a peak with height approximately 5 times the baseline noise. A chromatogram of the detection limit of the analytical procedure is shown in Figure 4.1.
4.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 2.34 µg per sample (0.005 ppm, 0.031 mg/m3). This is the amount of analyte spiked on the sampling device which allows recovery of an amount equivalent to the detection limit of the analytical procedure. Six veratrylamine-coated glass fiber filters were each liquid spiked with PHVA equivalent to 2.34 µg of phthalic anhydride (10 µL of a 234.4 µg/mL solution). The samples were stored at room temperature and extracted 4 h later with 4.0 mL of the extraction solvent. The injection size listed in the analytical procedure (5 µL) was used in the determination of the detection limit of the overall procedure.
Detection Limit of the Overall Procedure
|
| |||
| sample | theoretical amount | amount recovered | Recovery |
| number | (µg) | (µg) | (%) |
|
| |||
| 1 | 2.34 | 2.24 | 95.7 |
| 2 | 2.34 | 1.68 | 71.8 |
| 3 | 2.34 | 1.91 | 81.6 |
| 4 | 2.34 | 1.96 | 83.8 |
| 5 | 2.34 | 1.75 | 74.8 |
| 6 | 2.34 | 2.80 | 119.7 |
|
| |||
4.3. Reliable quantitation limit
The reliable quantitation limit is 3.59 µg per sample (0.008 ppm, 0.048 mg/m3). Because the recovery data in Section 4.2. did not meet the requirement of the precision (±1.96 SD) being ±25% or better, the experiment was repeated at a higher level (14 µL of a 256.7 µg/mL solution).
Reliable Quantitation Limit
|
| |||
| sample | amount spiked | amount recovered | recovery |
| no. | (µg) | (µg) | (%) |
|
| |||
| 1 | 3.59 | 3.08 | 85.8 |
| 2 | 3.59 | 3.13 | 87.2 |
| 3 | 3.59 | 3.25 | 90.5 |
| 4 | 3.59 | 3.07 | 85.5 |
| 5 | 3.59 | 3.30 | 91.9 |
| 6 | 3.59 | 3.10 | 86.4 |
| = | 87.9% | ||
| SD | = | 2.7% | |
| Precision | = | ±(1.96)(2.7%) | |
| = | ±5.2% | ||
|
| |||
4.4. Instrument response
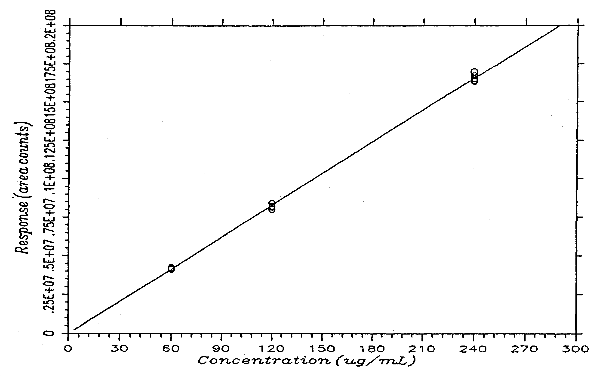
The instrument response to PHVA over the range of 0.5 to 2 times the target concentration is linear with a slope of 69142 area counts per microgram per milliliter. The response to PHVA at 200 nm was determined by multiple injections of analytical standards. The data are summarized in Table 4.4. and presented graphically in Figure 4.4.
Instrument Response to PHVA
|
| |||
| × target concn | 0.5× | 1× | 2× |
| µg/mL | 59.94 | 119.9 | 239.8 |
|
| |||
| area counts | 4201358 | 8381991 | 16337866 |
| 4160459 | 8136538 | 16403170 | |
| 4109023 | 8212463 | 16717588 | |
| 4113166 | 7993093 | 16986161 | |
| 4220818 | 8207300 | 16554520 | |
| 4244108 | 8125144 | 16491330 | |
| 4174822 | 8176088 | 16581772 | |
|
| |||
4.5. Storage data
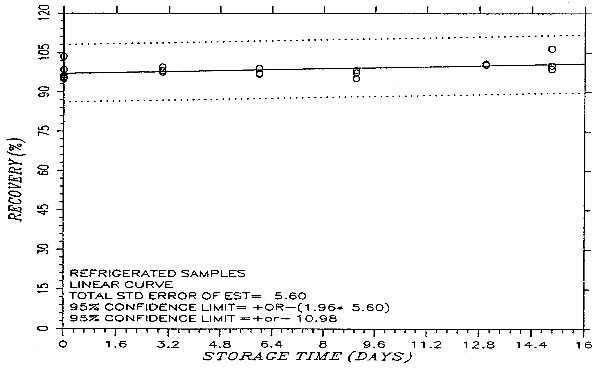
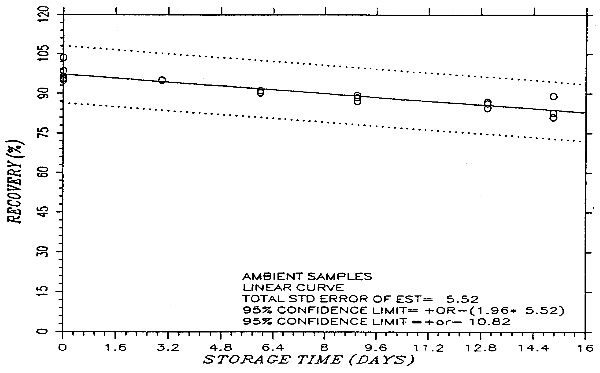
Thirty-six samples were prepared by liquid spiking PHVA at the target concentration. Humid air (80% RH) was pulled through the samples at 1.0 L/min for 75 min. Six samples were analyzed on the same day. Fifteen samples were stored in a refrigerator (0°C) and the other fifteen were stored in the dark at ambient temperature (20-25°C). Every few days over a 15-day period, three samples were selected from each of the two sets and analyzed. The results are listed in Table 4.5. and presented graphically in Figures 4.5.1. and 4.5.2.
Storage Test
|
| |||||||
| storage time | % recovery | % recovery | |||||
| (days) | (ambient) | (refrigerated) | |||||
|
| |||||||
| 0 | 95.4 | 95.8 | 103.6 | 95.4 | 95.8 | 103.6 | |
| 0 | 96.5 | 98.6 | 94.9 | 96.5 | 98.6 | 94.9 | |
| 3 | 94.9 | 95.2 | 95.3 | 98.2 | 97.6 | 99.5 | |
| 6 | 91.2 | 90.2 | 90.4 | 97.3 | 96.8 | 99.1 | |
| 9 | 87.1 | 88.5 | 89.4 | 97.3 | 98.3 | 95.2 | |
| 13 | 84.4 | 86.8 | 86.1 | 100.4 | 100.9 | 100.3 | |
| 15 | 80.9 | 82.6 | 89.0 | 106.5 | 98.8 | 100.1 | |
|
| |||||||
4.6. Precision (analytical procedure)
The precision of the analytical procedure is 0.015. The precision of the analytical procedure is defined as the pooled coefficient of variation determined from multiple injections of analytical standards representing 0.5, 1, and 2 times the target concentration (Section 4.4.).
Precision of the Analytical Procedure
(based on the data of Table 4.4.)
|
| |||
| × target concn | 0.5× | 1× | 2× |
|
| |||
| SD1 | 56494 | 128328 | 237608 |
| CV | 0.014 | 0.016 | 0.014 |
|
| |||
| 1 standard deviation in area counts | |||
4.7. Precision (overall procedure)
The precision of the overall procedure is determined from the storage data. The determination of the standard error of estimate (SEE) for a regression line plotted through the graphed storage data allows the inclusion of storage time as one of the factors affecting overall precision. The SEE is similar to the standard deviation except it is a measure of dispersion of data about a regression line instead of about a mean. It is determined with the following equation:
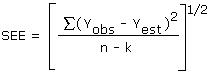
| where | n | = | total no. of data points |
| k | = | 2 for linear regression | |
| k | = | 3 for quadratic regression | |
| Yobs | = | observed % recovery at a given time | |
| Yest | = | estimated % recovery from the regression line at the same given time |
An additional ±5% for pump error is added to the SEE by the addition of variances. The precision at the 95% confidence level is obtained by multiplying the SEE (with pump error included) by 1.96 (the z-statistic from the standard normal distribution at the 95% confidence level). The 95% confidence intervals are drawn about their respective regression lines in the storage graphs as shown in Figure 4.5.2. The precision of the overall procedure of ±10.8% was obtained from Figure 4.5.2.
4.8. Reproducibility data
Six samples, prepared by liquid-spiking a known amount of PHVA, and a draft copy of this method were given to a chemist unassociated with this evaluation. The samples were stored for 11 days at about 0°C before being analyzed. No sample result had a percent deviation greater than the precision of the overall procedure.
Reproducibility Data
|
| ||||
| sample no. | µg found | µg expected | % found | % deviation |
|
| ||||
| 1 | 268.7 | 281.0 | 95.6 | -4.4 |
| 2 | 279.8 | 281.0 | 99.6 | -0.4 |
| 3 | 269.5 | 281.0 | 95.9 | -4.1 |
| 4 | 254.2 | 281.0 | 90.5 | -9.5 |
| 5 | 281.4 | 281.0 | 100.1 | +0.1 |
| 6 | 272.5 | 281.0 | 97.0 | -3.0 |
|
| ||||
4.9. Sampler capacity
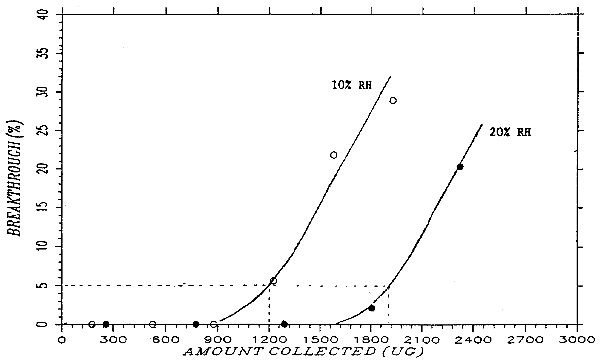
A test atmosphere of phthalic anhydride was generated by drawing air of controlled humidity through a U-tube that contained granules of phthalic anhydride. The U-tube was submerged in an oil bath maintained at 48°C. The test atmosphere passed through a cylindrical chamber (33 mm i.d. × 160 mm) and through two veratrylamine-treated glass fiber filters separated by spacers. At 30 minutes intervals, the air flow was stopped, the back filter was replaced with a new one, and the sampling resumed. This was repeated six times. The flow rate was 1.0 L/min. At the end of the experiment, the filters were analyzed and the upstream and downstream concentrations of phthalic anhydride calculated. The ratio of the downstream air concentration to the upstream concentration was defined as breakthrough. Many experiments were run, with varying flow rate, veratrylamine loading, and humidity. Sampler capacity improved with increased veratrylamine loading, decreasing flow rate, and most importantly, increasing humidity. A relative humidity of 10% at 24.5°C (dew point of -8.0°C) and a flow rate of 1.0 L/min were selected as the testing conditions for the sampler capacity at its most conservative. The breakthrough under these conditions are summarized in Table 4.9. and plotted in Figure 4.9. Figure 4.9. also shows breakthrough curve at 20% relative humidity for comparison. Because the phthalic anhydride concentration of the test atmosphere varied slightly from run to run, "cumulative amount collected" was plotted on the x-axis so that the breakthrough curves of more than one run can be put on a single graph. The cumulative amount collected for each interval was calculated by multiplying the air concentration with the midpoint air volume. The 5% breakthrough point was reached in about 1200 µg, or 100 L at 12 mg/m3.
Breakthrough Data at 2× Target Concentration
|
| |||||
| sample | amt found | air vol. | mg/m3 | BT | cumulative |
| (µg) | (L) | (%) | amt (µg) | ||
|
| |||||
| 0-30 min | 0 | 30.63 | 0 | 0 | 175 |
| 30-60 min | 0 | 30.63 | 0 | 0 | 526 |
| 60-90 min | 0 | 30.63 | 0 | 0 | 876 |
| 90-120 min | 19.58 | 30.63 | 0.639 | 5.6 | 1226 |
| 120-150 min | 76.36 | 30.63 | 2.493 | 21.8 | 1577 |
| 150-180 min | 101.21 | 30.63 | 3.304 | 28.9 | 1927 |
| front filter | 1905.12 | ||||
| total | 2102.27 | 183.78 | 11.44 | ||
|
| |||||
| BT = breakthrough | |||||
4.10. Extraction efficiency and stability of extracted samples
- 4.10.1. Extraction efficiency
The extraction efficiency for PHVA was determined by analyzing six veratrylamine-impregnated glass fiber filters that had been liquid-spiked with PHVA at the target concentration. These samples were stored at ambient temperature overnight before the extraction and analysis. The average extraction efficiency was 99.1%.
Extraction Efficiency
|
| |||
| sample no. | µg spiked | µg recovered | % recovery |
|
| |||
| 1 | 451.6 | 446.2 | 98.8 |
| 2 | 451.6 | 448.6 | 99.3 |
| 3 | 451.6 | 446.4 | 98.8 |
| 4 | 451.6 | 447.2 | 99.0 |
| 5 | 451.6 | 446.8 | 98.9 |
| 6 | 451.6 | 450.6 | 99.8 |
| 99.1 | |||
|
| |||
4.10.2. Stability of the extracted samples
The stability of the extracted samples was investigated by reanalyzing the extracted samples with fresh standards 1 day after the original analysis. The samples had been recapped and stored at room temperature. The change in the recovery averaged -0.9%.
Stability of Extracted Samples
|
| ||
| initial | recovery after | change |
| recovery (%) | 1 day (%) | (%) |
|
| ||
| 98.8 | 98.4 | -0.4 |
| 99.3 | 98.8 | -0.5 |
| 98.8 | 97.9 | -0.9 |
| 99.0 | 98.3 | -0.7 |
| 98.9 | 98.1 | -0.8 |
| 99.8 | 97.9 | -1.9 |
|
| ||
4.11. Chromatograms
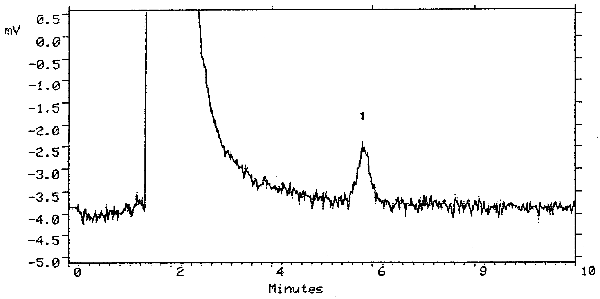
A chromatogram at the detection limit of the analytical procedure is shown in Figure 4.1. and a chromatogram of one of the 15-day refrigerated samples is shown in Figure 3.5.1.
4.12. Synthesis of PHVA
- 4.12.1. Reagents
Veratrylamine, 97%, from Aldrich
Phthalic anhydride, 99+%,
ACS reagent, from Aldrich
Toluene, b&j Brand, High Purity
Solvent, from Baxter
Isooctane, Optima, from Fisher Scientific
Chloroform, Optima, from Fisher Scientific
4.12.2. Apparatus
Erlenmeyer flasks
Filtering flask
Fritted-glass filtering
funnel
Explosion-proof hot plate
4.12.3. Procedure
Add 1.48 g (0.01 mole) of phthalic anhydride to a solution of 1.67 g (0.01 mole) of veratrylamine in 10 mL of chloroform. Stir the mixture for 10 min. Evaporate the chloroform in a hood. Dissolve the residue in a minimal amount of chloroform. While heating on a hot plate, slowly add isooctane until the solution just becomes cloudy. Clear the solution with an addition of a few drops of chloroform. Remove from the hot plate. After the solution has cooled to room temperature, store in a freezer overnight. Collect the crystals that formed. The melting point should be 120.0-121.5°C. The yield should be quantitative.
4.13. Cyclic imide of PHVA
- 4.13.1. Synthesis
Phthalic anhydride (99+%) 7.48 g (0.05 mole) and veratrylamine (97%) 8.62 g (0.05 mole) were dissolved in 150 mL of toluene and refluxed under a Dean Stark trap overnight. About 1.0 mL of water was collected. The solution was cooled to room temperature and the crystalline product was collected by filtration. The yield of the first crop was 11.65 g (78.4%); m.p. 149.5-150.5°C.
4.13.2. Comparison of the IR Spectra of PHVA and its cyclic imide
The IR spectra of PHVA and its cyclic imide were obtained from their nujol films. PHVA showed absorptions at 3340 cm-1 for the N-H stretching of the secondary amide functional group, 1695 cm-1 for the carbonyl stretching of the carboxyl group, and 1645 cm-1 for the carbonyl stretching of the secondary amide group (the "Amide I band"). The cyclic imide of PHVA, on the other hand, showed 1770 and 1700 cm-1 bands, indicative of the five-membered cyclic imide structure (Ref. 5.12.), and the 3340 cm-1 N-H stretching band is missing. The spectra are consistent with the structures.
5. References
- 5.1. National Institute for Occupational Safety and Health. Manual
of Analytical Methods, 2nd ed., Vol. 3, Method No. S179, U.S. Dept. of
Health, Education and Welfare, Washington, 1977, Publication No.
77-157-C.
5.2. Geyer, R. and Saunders, G.A., "Determination of Phthalic Anhydride in Workplace Air Using Reverse Phase High Performance Liquid Chromatography", J. Liq. Chromatography, 1986, 9 (10), 2281-2290.
5.3. Pfaeffli, P., "Phthalic Anhydride as an Impurity in Industrial Atmospheres: Assay in Air Samples by Gas Chromatography with Electron-capture Detection", Analyst, 1986, 111, 813-817.
5.4. Cram, D.J. and Hammond, G.S., "Organic Chemistry", 2nd ed., McGraw-Hill, New York, NY, 1964.
5.5. Mackison, F.W. et al., Eds., "NIOSH/OSHA Occupational Health Guidelines for Chemical Hazards", DHHS(NIOSH) Publication No. 81-123.
5.6. "Threshold Limit Values and Biological Exposure Indices for 1990-1991", American Conference of Governmental Industrial Hygienists, Cincinnati, OH, 1990.
5.7. "Code of Federal Regulations", Title 29, 1910.1000, Table Z-1-A. U.S. Government Printing Office, Washington, D.C., 1990.
5.8. Sweet, D.V., Ed., "Registry of Toxic Effects of Chemical Substances", 1985-86 ed., U.S. Department of Health and Human Services, Government Printing Office, DHHS(NIOSH) Publication No. 87-114.
5.9. Budavari, S., Ed., "Merck Index", 11th ed., Merck & Co., Rahway, NJ, 1989.
5.10. Grayson, M., Ed., "Kirk-Othmer Encyclopedia of Chemical Technology", 3rd ed., Vol. 17, John Wiley & Sons, New York, NY, 1982.
5.11. Author's personal observation.
5.12. Nakanishi, K., "Infrared Absorption Spectroscopy, Practical", Nankodo, Tokyo, Japan, 1962.